Tirzepatide Weight Loss: Key Takeaways From the SURMOUNT-1 Study
Tirzepatide Weight Loss: Key Takeaways From the SURMOUNT-1 Study
Table of Contents
I. What Are the SURMOUNT Trials?
III. Tirzepatide Weight Loss Results
IV. Tirzepatide and Cardiometabolic Risk Factors
Obesity and its related complications are a growing concern worldwide. For years, medical researchers have been working on developing safe and effective weight-loss medications.
Over the past few years, Eli Lilly has conducted the SURMOUNT clinical trials to test tirzepatide for weight loss, and the results are impressive.
The first of these trials, SURMOUNT-1, set out to determine whether tirzepatide could safely and effectively reduce body weight in adults without type 2 diabetes. And the results were impressive. Not only were the tirzepatide weight loss results significant, but tirzepatide also improved cardiometabolic risk factors. 1
In this article, we'll dive into the key takeaways of SURMOUNT-1 and explore just how safe and effective tirzepatide is for weight loss. We’ll break down tirzepatide weight loss results, its impact on cardiometabolic risk factors, and its safety profile.
What Are the SURMOUNT Trials?
The SURMOUNT trials represent a major milestone in evaluating tirzepatide for weight loss. This group of four clinical trials, which concluded in 2023, aimed to assess tirzepatide's effectiveness and safety across various populations struggling with obesity and excess weight. 2
- SURMOUNT-1 set out to determine if tirzepatide could effectively and safely reduce body weight in adults without type 2 diabetes. 1
- SURMOUNT-2 focused on tirzepatide weight loss results in overweight or obese adults with type 2 diabetes. 3
- SURMOUNT-3 took a different approach by having participants complete a 12-week intensive lifestyle intervention prior to starting tirzepatide. 4
- SURMOUNT-4 looked at the impact of stopping tirzepatide after initial weight loss. 4
An Overview of SURMOUNT-1
The SURMOUNT-1 clinical trial evaluated the effectiveness and safety of tirzepatide for weight loss in overweight or obese adults without diabetes. The study found that tirzepatide weight loss results were significant, and the medication was well-tolerated. 1
Researchers recruited over 2,500 participants for the 72-week trial. To qualify, participants had to have a:
- BMI greater than 30 (classified as obese)
OR
- BMI greater than 27 (classified as overweight) with the following weight-related health issues: hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease. 1
To focus solely on tirzepatide for weight loss, the study excluded people who already had diabetes or who had recently lost or gained a significant amount of weight. Participants also could not have taken any other weight loss medications in the past 3 months or undergone weight loss surgery. 1
Ultimately 2,539 qualified participants from several countries enrolled in the 72-week study. They were randomly divided into four groups:
- Tirzepatide 5 mg
- Tirzepatide 10 mg
- Tirzepatide 15 mg
- A placebo 1
The participants were given weekly injections of their assigned medication for 72 weeks, including a dose escalation period. Tirzepatide cannot be given at its full dose right away, so patients started with a small dose, which was then increased by 2.5 mg every 4 weeks until the assigned dosage was achieved. The dose escalation period lasted a maximum of 20 weeks, depending on the assigned dose. 1
To optimize tirzepatide weight loss results, all participants attended regular counseling sessions with nutritionists and healthcare providers. These lifestyle interventions encouraged:
- Healthy eating
- A 500-calorie daily deficit
- 150 minutes per week of physical activity 1
Tirzepatide Weight Loss Results

After 72 weeks, the data showed that tirzepatide weight loss results and body fat reduction were more substantial than the placebo.
- Tirzepatide 5 mg: Average body weight reduction of 15%.
- Tirzepatide 10 mg: Average body weight reduction of 19.5%.
- Tirzepatide 15 mg: Average body weight reduction of 20.9%.
- Placebo: Average body weight reduction of 3.1%. 1
These tirzepatide weight loss results are quite remarkable, considering that a 5% or greater reduction in weight is considered clinically meaningful in terms of improving health. Even at the lowest therapeutic dose of 5 mg, tirzepatide surpassed this threshold with an average 15% weight loss. 1
Researchers also looked at reductions in total body fat with tirzepatide compared to placebo. They found that those taking tirzepatide had an average body fat reduction of 33.9% versus only 8.2% for the placebo group. 1
Tirzepatide and Cardiometabolic Risk Factors
Tirzepatide weight loss results were remarkable in SURMOUNT-1, but researchers also took a close look at its impact on other cardiometabolic risk factors. They found that tirzepatide’s benefits go beyond the scale.
After 72 weeks of treatment, tirzepatide led to substantial reductions in waist circumference compared to the placebo:
- Tirzepatide 5 mg: waist reduction of 14 cm
- Tirzepatide 10 mg: waist reduction of 17.7 cm
- Tirzepatide 15 mg: waist reduction of 18.5 cm
- The placebo: waist reduction of 4 cm 1
The impact of tirzepatide on other cardiometabolic risk factors was equally compelling. Data analysis revealed substantial improvements compared to the placebo group, as illustrated in the chart below:
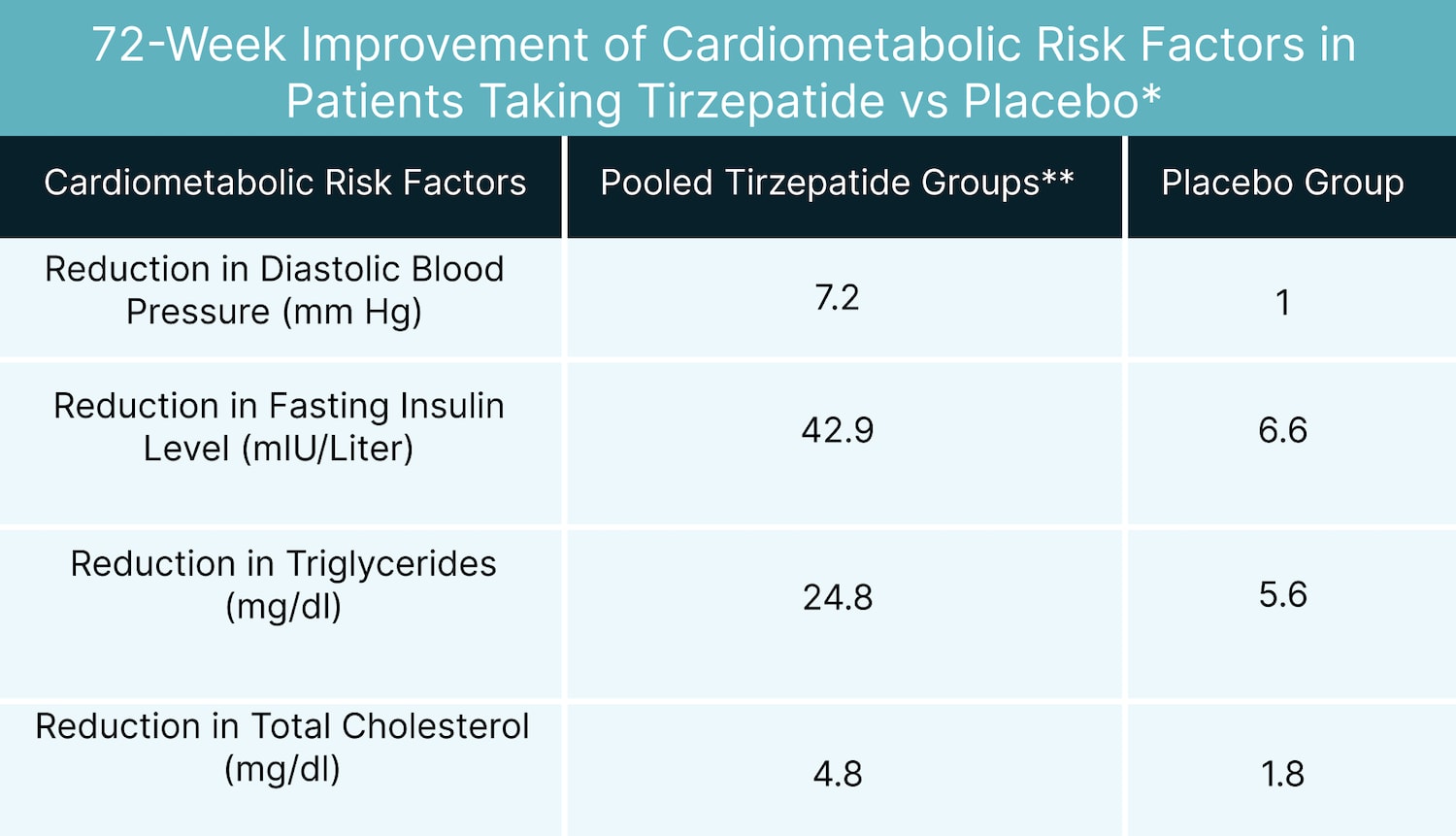
*1
**Pooled data from the groups taking tirzepatide 5 mg, 10 mg, and 15 mg
Tirzepatide Side Effects
While the tirzepatide weight loss results are remarkable, it’s also important to consider the safety profile of this medication.
Overall, 78.9 to 81.8% of the participants taking tirzepatide for weight loss reported at least one side effect during the treatment period. This is compared to 72% of participants in the placebo group. 1
The most frequently reported side effects were gastrointestinal issues such as:
- Nausea
- Diarrhea
- Constipation 1
These side effects occurred more often in the tirzepatide groups than placebo, were generally mild to moderate, and tended to subside after the initial dose escalation period. 1

More serious side effects occurred at similar rates in the tirzepatide and placebo groups. It is worth noting that approximately 21% of these serious events were linked to Covid-19, affecting all the participants in the study. 1
Conclusion
The SURMOUNT-1 clinical trial has shown that tirzepatide can be an effective medication for weight loss. Patients taking tirzepatide lost significant amounts of body weight and improved key cardiometabolic risk factors like waist circumference, lipids, blood pressure, and fasting insulin.
If you are considering tirzepatide for weight loss, know that lifestyle changes like reducing calories, eating healthy, and exercising were also critical to success in the SURMOUNT-1 trial. The tirzepatide weight loss results in this trial were optimized due to these lifestyle changes.
References
- Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., Kiyosue, A., Zhang, S., Liu, B., Bunck, M. C., & Stefanski, A. Tirzepatide once weekly for the treatment of obesity
- Eli Lilly and Company Lilly’s tirzepatide delivered up to 22.5% weight loss in adults with obesity or overweight in surmount-1
- Eli Lilly and Company Lilly’s surmount-2 results published in the Lancet show Tirzepatide achieved a mean weight reduction of 15.7% at the highest dose (15 mg) in adults with obesity or overweight and type 2 diabetes
- Eli Lilly and Company Tirzepatide demonstrated significant and superior weight loss compared to placebo in two pivotal studies
